Epidemiologic Perspective of Ovarian Cancer
Authors
INTRODUCTION
In the United States, ovarian cancer is the sixth most common cancer in women and the second most common malignancy of the female genital tract. The incidence of ovarian cancer increases with age, as follows:
Age <30: 5 in 100,000
Age 30–50: 21 in 100,000
Age 51–60: 37 in 100,000
Age >60: 46 in 100,0001 (incidence peaks at age 64)
Worldwide, the incidence of ovarian cancer varies among geographic regions. Ovarian cancer tends to occur among the most affluent women in the most highly industrialized countries. The incidence of ovarian cancer is significant in Scandinavia, Israel (American-born or European-born residents), and North America. The frequency of ovarian cancer is low among East Indians and Africans, and African Americans and Latinos in the United States. Although the incidence of ovarian cancer is lower in Japan and Hong Kong compared with the United States, in the 1990s it increased 15–25% every 5 years in both of these countries.2
The cause of ovarian cancer is unknown. Epidemiologic studies suggest that environmental or dietary factors or both may play a role. Advances in molecular biology suggest that genetics play a larger role than previously suspected. Despite major advances made in treatment, ovarian cancer continues to have the highest fatality-to-case ratio of all gynecologic malignancies.3 A better understanding of potential risk factors for the development of ovarian cancer may help prevent or at least decrease the incidence of this chronic, fatal disease.
FACTORS RELATED TO THE DEVELOPMENT OF OVARIAN CANCER
Hygienic perineal talc4
Because talc is related to asbestos, it has been implicated as a cause of ovarian cancer.5 Besides the chemical similarities between talc and asbestos, cosmetic talcum powder manufactured before 1976 contained significant amounts of the three types of asbestos: tremolite, chrysolite, and crocidolite.5, 6 Studies by Heller and associates5 and Henderson and colleagues7 showed that talc particles may be found in normal and malignant tissues. Conceptually, talcum powder applied to the perineum can reach the ovaries via retrograde flow. The association between talc and ovarian cancer remains equivocal. Whittemore and associates8 could not show an association between genital talcum powder exposure and ovarian cancer; however, Cramer and coworkers9 reported a relative risk (RR) of 1.9 (p <0.03) for ovarian cancer among talc users. The RR was increased further in women who used talcum powder on perineal napkins and dusting powder on the perineum (Table 1). Wong and coworkers10 reported, however, in a case–control study that included 704 controls and 430 cases of ovarian cancer no increased risk for ovarian cancer with the use of perineal talc. There was no increased risk with 20 or more years of use of perineal talc. In a meta-analysis of six studies, Harlow and colleagues11 calculated a modest risk for ovarian cancer development among talc users: RR 1.3 (95% confidence interval (CI) 1.1–2.0) (Table 2).11 Although these case–control studies showed a small increased risk for development of ovarian cancer with perineal talc use, in 2000 the scientific advisory panel to the National Toxicology Program, a branch of the National Institutes of Health, voted seven to three against recommending that perineal talc be listed as a human carcinogen.12
Table 1. Relative risks (RR) for common epithelial ovarian cancers associated with talc exposure in perineal hygiene
| Types of perineal exposure | |||||
| No perineal exposure | Any perineal exposure, but not on napkins | As dusting powder | On napkins, but not as dusting powder | Both on napkins and as dusting powder | |
| Cases | 123 (57.2%) | 92 (42.8%) | 43 (20.0%) | 17 (7.9%) | 32 (14.9%) |
| Controls | 154 (71.6%) | 61 (28.4%) | 34 (15.8%) | 14 (6.5%) | 13 (6.0%) |
| Crude RR | 1 | 1.89 | 1.58 | 1.52 | 3.08 |
| Adjusted RR* | — | 1.92 | | 1.55 | 3.28 |
| 95% CI | — | 1.27–2.89 | 1.68–6.42 | ||
CI, confidence interval.
*Adjusted for parity and menopausal status.
(Cramer DW, Welch WR, Scully RE et al: Ovarian cancer and talc: A case-control study. Cancer 50: 372, 1982)
Table 2. Odds ratio (OR) with 95% confidence intervals (CI) of ovarian cancer in relation to any perineal exposure to talc as reported in previous epidemiologic studies
Cases | Controls | |||||
Author (year) | Total | Talc exposure | Total | Talc exposure | Crude OR | 95% CI |
Cramer et al (1982) | 215 | 92 (42.8%) | 215 | 61 (28.4%) | 1.9 | 1.3–2.0 |
Hartge et al (1983) | 135 | 67 (49.6%) | 171 | 100 (58.5%) | 0.7 | 0.4–2.0 |
Whittemore et al (1988) | 188 | 98 (52.1%) | 539 | 248 (46.0%) | 1.4 | 0.9–2.0 |
Harlow et al (1989) | 116 | 49 (42.2%) | 158 | 64 (40.5%) | 1.1 | 0.7–2.0 |
Booth et al (1989) | 217 | 141 (65.0%) | 434 | 256 (59.0%) | 1.3 | 0.9–1.0 |
Harlow et al (1992) | 235 | 114 (48.5%) | 239 | 94 (39.3%) | 1.5 | 0.9–2.0 |
All studies | 1106 | 561 (50.7%) | 1756 | 823 (46.9%) | 1.3 | 1.1–2.0 |
CI, confidence interval.
(Harlow BL, Cramer DW, Bell DA, et al: Perineal exposure to talc and ovarian cancer risk. Obstet Gynecol 80: 19, 1992)
Diet
The major dietary distinction in industrialized nations is the high intake of meat and animal fat, which has been reported to be associated with an increased incidence of ovarian cancer in several studies.13 Shu and associates14 reported a significant (p <0.01) dose–response relationship between the intake of fat from animal sources and ovarian cancer risk in a case–control study of 172 epithelial ovarian cancer cases and 172 controls. In a prospective study of 20,305 women, Helzlsouer and colleagues15 showed that women with cholesterol levels greater than 200 mg/dL are at increased risk for ovarian cancer.
Lactose, found naturally only in milk, is the only source of dietary galactose. Ovarian tissue has a relative abundance of galactose-metabolizing enzymes, and experimental studies on rats indicated that galactose-rich diets can have an adverse effect on the ovaries.16, 17, 18, 19 Young women with galactosemia, an inborn error of galactose metabolism, experience hypergonadotropic hypogonadism and early ovarian failure.20 Cramer and colleagues21 proposed that increased dietary galactose consumption and low serum levels of galactose-1-phosphate uridyltransferase, an important enzyme in the metabolism of galactose, may be linked to an increased risk of ovarian cancer. In a case–control study, the investigators found that women who consumed significantly more yogurt and cottage cheese, the only foods consumed regularly that distinguished the cases from the controls, had three times the incidence of ovarian cancer than the controls. Women with ovarian cancer were reported to have significantly lower galactose-1-phosphate uridyltransferase levels than controls (Table 3). These authors proposed that in women with an inherited abnormality of galactose-1-phosphate uridyltransferase, the ovaries would be exposed to galactose for a prolonged period, increasing the ovarian cancer risk in these women compared with women who can metabolize galactose efficiently.
Table 3. Risks for ovarian cancer by lactose consumption and transferase activity
No. of subjects (%) | ||||
Lactose consumption (g/day) | Combination transferase activity | Cases (n = 145) | Controls (n = 127) | RR (95% CI) |
≤11 | ≥22.9 | 29 (20) | 36 (28) | 1.0 |
≤11 | <22.9 | 30 (21) | 35 (28) | 1.1 (0.5–2.2) |
>11 | ≥22.9 | 33 (23) | 29 (23) | 1.3 (0.6–2.7) |
>11 | <22.9 | 53 (36) | 27 (21) | 2.2 (1.1–4.5) |
Cl, confidence interval; RR, relative risk.
(Cramer DW, Harlow BL, Willett WC, et al: Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet 2: 66, 1989)
Several other studies found no association between galactose consumption and the incidence of ovarian cancer. In a case–control study of 450 patients, Risch and associates22 showed that galactose consumption did not increase the risk of epithelial ovarian cancer (Table 4). In a 2000 report of the Nurses’ Health Study of 80,326 women who completed a dietary questionnaire, Fairfield and coworkers23 reported, however, a 44% greater risk for the development of invasive epithelial ovarian cancer for women with the highest consumption of lactose compared with women with the lowest consumption (RR, 1.44; 95% CI, 1.01–2.07; p for trend 0.07). The largest contributors to dietary lactose were skim and low-fat milk.3 Another, perhaps better relationship between milk consumption and ovarian cancer was proposed by Mettlin and Piver,24 who evaluated this association in terms of animal fat content in milk. The RR for ovarian cancer was 3.1 for women drinking more than one glass of whole milk each day compared with women who never drank whole milk (Table 5). Women who drank only whole milk were at increased risk for ovarian cancer compared with women who drank skim or 2% fat milk. These findings suggest that animal fat, described as the fat content in milk, may be the source of increased risk. In a report of 2008, Prentice and colleagues25 analyzed data from the randomized controlled Dietary Modification trial of a total of 50,000 women to see whether changes in women's diets decreased the risk of ovarian and endometrial cancer and invasive cancer overall. In this trial, nearly 20,000 women were randomly assigned to the diet modification group and almost 30,000 women ate their normal diet. Those who followed a low-fat diet for 8 years had a reduced incidence of ovarian cancer among postmenopausal women.
Table 4. Trends in risk of ovarian cancer per unit intake of dairy nutrients and selected items, Southern Ontario, 1989–92
Adjusted odds ratio (95% confidence interval) | |||
Nutrient/item | Unit/day (g) | Model 1 | Model 2 |
Lactose | |||
All subjects | 10 | 1.03 (0.93–1.12) | 0.99 (0.90–1.09) |
Subjects not reporting lactose intolerance | 10 | 1.02 (0.93–1.13) | 0.99 (0.90–1.09) |
Subjects reporting lactose intolerance | 10 | 0.89 (0.64–1.25) | 0.84 (0.60–1.18) |
Subjects who never used OC | 10 | 1.11 (0.97–1.27) | 1.06 (0.93–1.22) |
Subjects who ever used OC | 10 | 0.98 (0.89–1.09) | 0.95 (0.85–1.06) |
Whole milk | 300 | 1.20 (0.80–1.80) | 0.98 (0.64–1.49) |
2% milk | 300 | 0.99 (0.85–1.15) | 0.92 (0.79–1.08) |
Skim milk | 300 | 1.04 (0.84–1.29) | 1.08 (0.87–1.35) |
Yogurt | 30 | 1.02 (0.96–1.09) | 1.03 (0.97–1.10) |
OC, oral contraceptives.
(Adapted from Risch HA, Jain M. Marrett LD, et al: Dietary lactose intake, lactose intolerance, and the risk of epithelial ovarian cancer in Southern Ontario [Canada]. Cancer Causes Control 5: 540, 1994)
Table 5. Ovarian cancer and whole milk consumption
Frequency (total) | Ovarian cancer (n = 297l) | Controls (n = 587) | Relative risk |
Never | 174 | 410 | 1 |
Less than one glass daily | 62 | 109 | 1.3 |
One glass daily | 25 | 41 | 1.4 |
More than one glass daily | 36 | 27 | 3.1 |
p <0.001 |
(Adapted from Mettlin C, Piver MS: A case-control study of milk drinking and ovarian cancer risk. Am J Epidemiol 132: 873, 1990)
Infertility and fertility drugs
Infertility and the use of fertility drugs have been found to increase the risk of ovarian cancer.26, 27, 28, 29 In a case–control study of 3837 women evaluated for infertility, Rossing and colleagues26 observed an increased ovarian cancer risk among infertile women (standardized incidence ratio (SIR) 2.5; 95% CI,1.3–4.5); this study suggested that women with infertility resulting from abnormalities in ovulation were at greater risk for ovarian cancer (SIR 3.7; 95% CI 1.4–8.1) than women with infertility resulting from tubal abnormalities (SIR 3.0; 95% CI 0.4–10.8). Similarly, Whittemore and coworkers27 reported that it is not the estimated number of years of ovulation, the number of pregnancies, or the lack of use of oral contraceptives that increases a woman’s risk for ovarian cancer, but rather the inability to conceive among ovulating women who have sexual intercourse for 10 or more years, unprotected by any type of contraception (Table 6).
Table 6. Ovarian cancer risk of unprotected intercourse
Years of unprotected intercourse | Ovarian cancer | Controls | Relative risk | p Value |
<2 | 91 (50%) | 323 (62%) | 1 |
|
2–9 | 48 (26%) | 117 (22%) | 1.5 | 0.08 |
>10 | 43 (24%) | 82 (16%) | 1.8 | 0.01 |
(Adapted from Whittemore AS, Wu ML, Paffenbarger RS, et al: Epithelial ovarian cancer and the ability to conceive. Cancer Res 49: 4047, 1989)
The use of ovulation-inducing agents is increasing in the United States; in the 1980s and 1990s, the number of clomiphene citrate prescriptions dispensed annually nearly doubled.29 In the late 1990s, researchers raised concerns regarding the relationship between ovulation-inducing agents and the development of ovarian cancer. Rossing and colleagues reported26 an increased risk of ovarian cancer among women who had ever used clomiphene citrate (SIR 3.1; 95% CI 1.4–5.9) and a further increase in risk of ovarian cancer among women who used clomiphene for 12 or more cycles (SIR 11.1; 95% CI 1.5–82.3). In a nationwide case–control study from Israel, Shushan and associates29 reported an adjusted odds ratio (OR) of 3.19 (95% CI 0.86–11.82) among women who used human menopausal gonadotropins alone and an adjusted OR of 1.42 (95% CI 0.65–3.12) among women who used human menopausal gonadotropins alone or combined with clomiphene citrate. In an analysis of fertility drug use from eight case–control studies conducted between 1989 and 1999 in the United States, Denmark, Canada, and Australia, Ness and coworkers30 reported, however, no increased risk for the development of invasive epithelial ovarian cancer among infertile women taking clomiphene citrate or human menopausal gonodotropin.
Estrogen replacement therapy
Estrogen replacement therapy (ERT) has been used to relieve menopausal symptoms such as vaginal dryness and hot flashes. The association between ERT and ovarian cancer is inconclusive, however.34, 35, 36, 37, 38, 39 Results from the Collaborative Ovarian Cancer Group showed no risk of ovarian cancer among ERT recipients (OR 1.1; 95% CI 0.59–2.0), and several studies agreed with these findings.34, 39 Cramer and colleagues37 reported an association, although not statistically significant, between ERT and the development of ovarian cancer (RR 1.56; 95% CI 0.85–2.87). The most controversial report on ERT and ovarian cancer is that of the Department of Epidemiology and Surveillance Research of the American Cancer Society.40 These investigators did not report on the association between the use of ERT and the risk of developing ovarian cancer but rather that among postmenopausal women, the use of ERT in the 1970s and early 1980s for 10 or more years doubled the risk of dying from ovarian cancer. The authors assumed that women took estrogen alone (without progesterone), although they did not know which estrogen product the women took. In a 1979–1998 cohort study of a total 44,241 postmenopausal women, Lacey and colleagues 41 reported that women who used estrogen-only replacement therapy for 10 or more years were at significantly increased risk of ovarian cancer (RR for 10–19 years, 1.8; RR for 20 or more years, 3.2 ). In the UK Million Women study42 almost 1 million postmenopausal women (total 948,576) were followed to evaluate the effect of HRT on women's risk of developing and dying from ovarian cancer. Current HRT users in this study were significantly more likely to develop and die from ovarian cancer than never users (RR 1.20 for incident disease; RR 1.23 for death ) (Table 7).
The Women’s Health Initiative randomized controlled trial of 2002 randomized women aged 50–79 years with an intact uterus to conjugated equine estrogen plus medroxyprogesterone acetate or placebo. In the combination estrogen–progesterone group there was a nonsignificant increase in ovarian cancer (HR 1.6, 95% CI 0.8–3.2); 42 versus 27 cases per hundred thousand person years in the estrogen–progesterone group versus placebo.43
In contrast, the 2002 report by Lacey and co-workers of a prospective study of 44,241 postmenopausal women (mean age 56.6 years) who participated in the Breast Cancer Detection Demonstration Project, reported that the duration of estrogen-only therapy was significantly associated with the development of ovarian cancer: RR for 10–19 years and more than 20 years were 1.8 (95% CI 1.1–3.0) and 3.2 (95% 1.7–5.7) increase in RR per year of use of estrogen-only HRT.44
Although the issue of HRT with estrogen plus progesterone or estrogen alone as causative of ovarian cancer is far from settled, the fact that many tumors have high levels of estrogen receptors with increased proliferation may be the explanation why HRT alone significantly increases women’s risk for developing ovarian cancer.
Table 7. Relative risk of incidence and mortality of ovarian cancer by use of HRT
| Cases/Population | Deaths | |||
| Last reported HRT use | Number (1000s) | Relative risk (95% CI) | Number | Relative risk (95% CI) |
| Never users | 1142/474.7 | 1.00 | 819 | 1.00 |
| Past users | 391/186.8 | 0.98 (0.88–1.11) | 275 | 0.97 (0.84–1.11) |
| All current users | 740/287.1 | 1.20 (1.09–1.32) | 497 | 1.23 (1.09–1.38) |
| Estrogen-only | 242/85.9 | 1.34 (1.13–1.60) | 167 | 1.48 (1.20–1.81) |
| Estrogen + progestagen | 414/169.3 | 1.14 (1.01–1.28) | 276 | 1.15 (1.00–1.33) |
| Other | 84/31.9 | 1.22 (0.98–1.53) | 54 | 1.14 (0.87–1.51) |
(Adapted from Million Women Study Collaborators: Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 2007, 369: 1703)
In meta-analysis of eight cohort and 19 case–control studies, Zhou and colleagues45 found that the risk for ovarian cancer was higher among current users of more than 5 years than among those of less than 5 years in cohort studies. However, they did not find an association between long-term or ever use of HRT and ovarian cancer risk based on data from six case–control studies.
Genetic factors
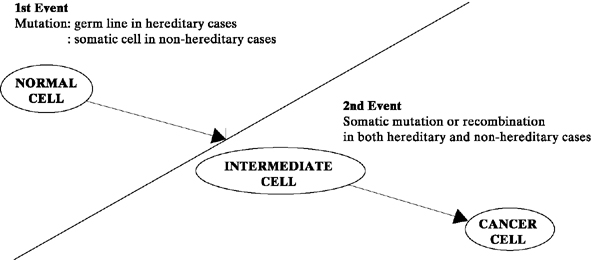
It is commonly accepted that cancer results from a series of genetic alterations.46, 47 In 1972, Knudson and Strong48 proposed the two-hit mutation model for the development of cancer (Fig. 1). According to this concept, in inherited cancers, the first mutation (single-gene alteration) is prezygotic, making the fetus predisposed to the development of cancer from conception onward. Cancer develops in the somatic cells (in this case, the ovary), however, only when a postzygotic second mutation occurs after birth. In contrast, nonhereditary cancers occur when both mutations occur postzygotically in the somatic cell line (the ovary). Knudson and Strong’s hypothesis helps explain why hereditary cancers frequently occur 10–20 years earlier than nonhereditary cancers (i.e., because the first mutation has occurred prezygotically).
|
Genetic changes in two categories of normal cellular genes – proto-oncogenes and tumor-suppressor genes – have been proposed to cause cancer.46, 47, 49 Genetic changes (e.g., point mutations, translocations, amplifications) in proto-oncogenes facilitate the transformation of a normal cell to a malignant cell by producing an altered or overexpressed gene product. Products of proto-oncogenes include cellular growth factors, growth factor receptors, enzymes, and DNA-binding proteins.50, 51 Tumor-suppressor genes are proposed to prevent cancer.47, 49, 50, 51, 52, 53 Inactivation or loss of these genes contributes to the development of cancer because of the lack of a functional gene product. Dysfunction of tumor-suppressor genes could result in abnormal cell division, abnormal gene expression, or the increased ability of cells in tissue to detach.
Activation of several proto-oncogenes (particularly K-ras, h-ras, c-myc, and HER-2/neu) has been assayed by various techniques in ovarian tumors and in established ovarian tumor cell lines.54, 55, 56 These techniques have measured the ability of isolated tumor DNA to transform the murine fibroblast cell line (NIH/3T3), and the amplification of proto-oncogene messenger RNA and protein by tumors; data from these investigations showed that activation of proto-oncogenes occurs in ovarian tumors. The significance of proto-oncogene activation and the role, if any, it plays in the development of ovarian cancer remain to be determined.
Until relatively recently, there was little evidence in the literature that the origin of some ovarian cancers was either genetic or familial. In 1979, we reported a family in which epithelial ovarian cancer developed in five members, spanning three generations: a grandmother, three daughters, and a granddaughter.57 Shortly thereafter, five members of a second family, spanning two generations (three sisters, a first cousin, and her daughter), came under our care for epithelial ovarian cancer.58 Our review of the literature revealed that before 1970, familial ovarian cancer had been reported in only five families. In contrast, during the 1980s, 26 more families were documented as having familial ovarian cancer. In response to this seemingly real increase in the incidence of familial ovarian cancer, and because of the need for appropriate genetic counseling, a Familial Ovarian Cancer Registry was established at Roswell Park Cancer Institute in 1981 by one of us (Piver MS) to document the number of cases occurring in the United States and to study the mode of inheritance. It was postulated that if an autosomal dominant mode of transmission was established, genetic counseling for prophylactic oophorectomy at an appropriate age might lead to a decrease in the death rate from ovarian cancer in such families.
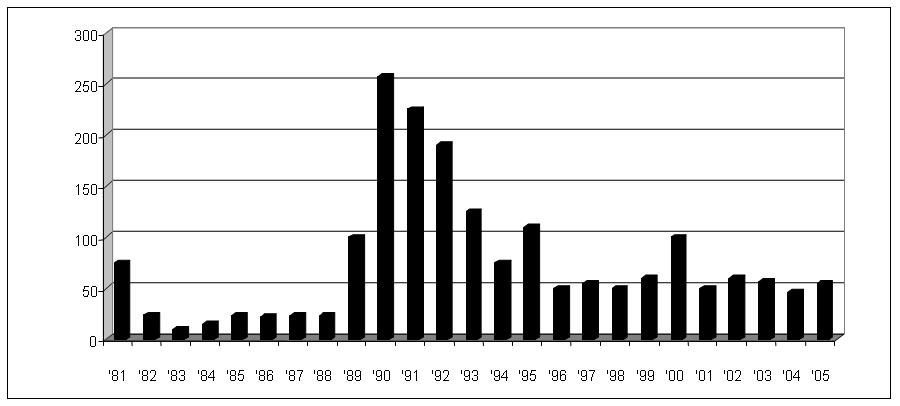
The Registry’s first report in 1984 documented 201 cases of ovarian cancer in 94 families.59 After the initial collection of 76 cases in 1981, the average number of cases enrolled between 1982 and 1996 was 189 per year (range 32–635). Because of an increased awareness of a possible genetic link generated by the media, the Registry now receives daily information on new families with familial ovarian cancer. In 1990, the Registry was renamed the Gilda Radner Familial Ovarian Cancer Registry (GRFOCR) in honor of the comedic actress, Gilda Radner, who died of ovarian cancer. Up to December 31, 2001, the Registry had enrolled 1652 families with two or more close relatives with ovarian cancer (Fig. 2). The Registry has made it clear that familial ovarian cancer is not a rare occurrence, as originally conceived in the four decades from 1930 to 1970.
Three conditions are associated with familial ovarian cancer: (1) site-specific ovarian cancer, in which the familial risk is restricted to ovarian cancer; (2) breast/ovarian cancer syndrome, with clustering of ovarian and breast cancer cases in extended pedigrees; and (3) cancer syndrome family, also known as Lynch syndrome II, which consists of hereditary nonpolyposis colorectal cancer (HNPCC) (with most of the cancers occurring in the proximal colon) in association with ovarian or endometrial carcinoma or both.60 In all of these syndromes, inheritance seems to follow an autosomal dominant pattern with variable penetrance. In addition, several genetic factors are associated with increased gonadal dysgenesis (dysgerminoma),61 Peutz-Jeghers syndrome (mucocutaneous pigmentation or gastrointestinal polyposis, granulosa cell tumor, and cystadenoma),62 and basal cell nevus syndrome (fibroma).63
Hall and associates64 in 1990 mapped by genetic linkage a locus for familial ovarian cancer to the long arm of chromosome 17 in the interval 17q12-21. Before the cloning of BRCA1 by Miki and coworkers65 in 1994, the GRFOCR participated in the Breast Cancer Linkage Consortium Study of 145 breast/ovarian cancer families.66 These families consisted of three or more cases of early onset (<60 years old) breast or ovarian cancer. It was estimated that 92% of the families in which there was no male breast cancer and with two or more cases of ovarian cancer were linked to BRCA1. The GRFOCR participated in another international study of nine families with site-specific ovarian cancer defined as three or more cases of epithelial ovarian cancer and no case of breast cancer diagnosed at less than 50 years of age. This study documented that 89% of such site-specific ovarian cancer families are linked to BRCA1.67, 68
In 1995, Wooster et al.69 cloned BRCA2, which previously had been mapped by genetic linkage to chromosome 13q12. It is now estimated that BRCA1 and BRCA2 are responsible for 5–10% of all epithelial ovarian cancers with 52% BRCA1, 32% BRCA2, and 16% other yet undefined genes.70 In the United States, lifetime risk of developing epithelial ovarian cancer is 1 in 55 or 1.8% compared with 28–44% for BRCA1 carriers and 27% for BRCA2 carriers.
The HNPCC patients are at increased risk of colorectal cancer, endometrial cancer, and also ovarian cancer (12% life-time risk).71 Defective mismatch repair (MMR) is a hallmark of the HNPCC syndrome and is found in more than 95% of HNPCC-associated tumors. About 2% of ovarian cancer is caused by germline mutations in the MMR genes (MLH1, PMS2, MSH2, and MSH6). Malanda et al.72 analyzed expression of MMR proteins in HNPCC patients and concluded that ovarian cancer patients with a family history suggestive of HNPCC, shoud be ascertained for MMR defects.
Because familial ovarian cancer (site-specific) is thought to be inherited in an autosomal dominant pattern with variable penetrance, sisters and daughters in families with a history of familial ovarian cancer (two or more first-degree relatives, mother/sister/daughter, who share one half of their genes) may have a 50% chance of disease development. This figure compares with a 1.8% (1 in 55) in women without a family history. It has been recommended that such women undergo a prophylactic oophorectomy by age 35 if they have completed childbearing. A word of caution: There have been reports of women with a family history of ovarian cancer who have been diagnosed with intra-abdominal carcinomatosis histologically similar in appearance to ovarian adenocarcinoma (also known as extraovarian papillary carcinoma of the peritoneum) after prophylactic oophorectomy.73 In some cases, the prophylactically removed ovaries were cancerous on retrospective review;74 however, in other cases of extraovarian papillary carcinoma, the prophylactically removed ovaries were cancer-free. It is postulated that whatever causes ovarian cancer also affects the peritoneum to form an extraovarian papillary carcinoma. From 1981 to July 1982, the GRFOCR had enrolled 931 families totaling 1222 cases of familial ovarian cancer. Of these, 324 had undergone prophylactic oophorectomy.75 Of these 324 women, six or 1.8% developed primary peritoneal carcinoma indistinguishable from epithelial ovarian cancer 1, 2, 5, 13, 15, and 27 years after prophylactic oophorectomy. Although these data suggest that development of primary peritoneal carcinoma after prophylactic oophorectomy for a family history of familial ovarian cancer is not a common event, they do point out that all women who undergo such preventive surgery need to be under lifetime surveillance for the possible development of primary peritoneal carcinoma.
FACTORS PROTECTIVE AGAINST OVARIAN CANCER
Oral contraceptives
Epidemiologic studies have shown repeatedly that the use of oral contraceptives reduces a woman’s risk for epithelial ovarian cancer. Whittemore and associates34 showed that women who used oral contraceptives had a RR of 0.66 (95% CI 0.55–0.78), a 40% reduction compared with population controls. The risk of ovarian cancer decreases as the duration of using oral contraceptives increases, and this protective effect occurs even with a short duration of use. Using meta-analysis and regression equations, Gross and Schlesselman76 proposed that women with a positive family history of ovarian cancer can reduce their risk to a level below that of a woman with no family history of ovarian cancer and who has never used oral contraceptives. Similarly a nulliparous woman who uses oral contraceptives for 5 years can reduce her risk to the level of a parous woman who has never used oral contraceptives (Table 8).
Table 8. Cumulative number of cases of epithelial ovarian cancer per 100,000 women
| Group | Years of OC use | |||
Never | 1 | 5 | 10 | |
Positive family history | ||||
Age 40 | 218 | 165 | 111 | 95 |
Age 50 | 644 | 475 | 307 | 248 |
Age 55 | 1007 | 736 | 471 | 377 |
Negative family history | ||||
Age 40 | 100 | 76 | 51 | 44 |
Age 50 | 298 | 219 | 142 | 115 |
Age 55 | 466 | 340 | 218 | 174 |
Parous | ||||
Age 40 | 82 | 62 | 41 | 35 |
Age 50 | 270 | 199 | 128 | 102 |
Age 55 | 429 | 313 | 199 | 158 |
Nulliparous | ||||
Age 40 | 165 | 125 | 83 | 70 |
Age 50 | 543 | 399 | 256 | 206 |
Age 55 | 862 | 628 | 401 | 31 |
OC, oral contraceptive.
(Adapted from Gross TP, Schlesselman JJ: The estimated effect of oral contraceptive use on the cumulative risk of epithelial ovarian cancer. Obstet Gynecol 83: 419, 1994)
In 1998, Narod and Risch et al.77 determined that oral contraceptive use for more than 6 years' accumulative use reduced the risk of ovarian cancer by 60% among women who carry deleterious mutations of the genes BRCA1 or BRCA2. The data from study of Whittmore et al.78 also support the hypothesis that long-term oral contraceptive use reduces the risk of ovarian cancer among women who carry mutations of BRCA1 or BRCA2. The risk decreased by 5% (1–9%) with each year of use (p = 0.01) and use for 6 or more years was associated with an odds ratio of 0.62 (0.35–1.09) (Table 9).
Table 9. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations
| Years of use | Decreased risk cases (147) vs. controls (304) |
| <1 | None |
| 3–5 | 31% |
| 6+ | 38% |
(Adapted from Whittemore AS, Balise RR, Pharoah PDP, et al: Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer 91: 1911, 2004)
Pregnancy
Pregnancy consistently has been found to have a protective effect against ovarian cancer.34, 79, 80 Data from the Collaborative Ovarian Cancer Group showed a 40% risk reduction after the first term pregnancy and a 14% risk reduction with each successive pregnancy (Table 10).34 In a large prospective study of 121,700 women in the Nurses’ Health Study, Hankinson and coworkers81 documented that parous women have a reduced ovarian cancer risk relative to nulliparous women (RR 0.84; 95% CI 0.77–0.91 per pregnancy). Whittemore and associates34 also showed that failed pregnancies (defined as miscarriages, abortions, ectopic pregnancies, and stillbirths) also decreased the risk of ovarian cancer but had a lesser effect than that of full-term live births.
Table 10. Odds ratio (OR) for invasive epithelial ovarian cancer according to parity
| No. of term pregnancies* | Cases | Controls | |||||
No. | % | No. | % | OR† | 95% CI | p Value | |
0 | 322 | 24 | 765 | 14 | 1.0 |
|
|
1 | 164 | 12 | 605 | 11 | 0.60 | 0.47–0.76 | <0.001 |
2 | 376 | 28 | 1515 | 27 | 0.53 | 0.44–0.64 | <0.001 |
3 | 265 | 19 | 1259 | 22 | 0.48 | 0.39–0.59 | <0.001 |
4 | 135 | 10 | 774 | 14 | 0.36 | 0.28–0.46 | <0.001 |
5 | 56 | 4 | 345 | 6 | 0.33 | 0.23–0.46 | <0.001 |
6 | 45 | 3 | 346 | 6 | 0.29 | 0.20–0.42 | <0.001 |
Any term pregnancy | 1041 | 76 | 4844 | 86 | 0.47 | 0.40–0.56 | <0.001 |
CI, confidence interval.
*Pregnancies of at least 20 weeks’ gestation.
†Adjusted for age, study, and oral contraceptive use.
(Adapted from Whittemore AS, Harris R, Itnyre J, and the Collaborative Ovarian Cancer Group: Characteristics relating to ovarian cancer risk: Collaborative analysis of 12 US case-control studies: Part II. Invasive epithelial ovarian cancer in white women. Am J Epidemiol 136: 1184, 1992)
Two theories have been offered to explain the decreased incidence of ovarian cancer associated with oral contraceptive use and multiparity or, conversely, why nulliparous women and, more significantly, women who have tried to conceive but have failed are at higher risk for ovarian cancer. In 1948, Garder82 proposed the concept termed by Cramer and Welch83 the excess gonadotropin secretion theory. This theory held that ovarian cancer results from continually high levels of gonadotropin acting on the ovaries as a result of ovarian failure or the inability of the ovarian–pituitary feedback mechanism to inhibit the excretion of these high levels of gonadotropin on the ovary. The incessant ovulation theory of Fathala84 proposed that continuous ovulation causes repeated trauma to the ovary and leads to the development of ovarian cancer, noting that the risk increases in proportion to the number of times a woman ovulated in her lifetime. Conversely, most factors that are associated with a lower risk of ovarian cancer (e.g., oral contraceptive use, pregnancy) suppress ovulation. Berchuck and colleagues85 showed that patients with ovarian cancer who had an overexpression of p53 had a significantly greater number of lifetime ovulations (OR 7.7; 95% CI 1.4–41.2); these results strengthen the hypothesis that proliferation of ovarian epithelium in response to ovulation may increase the risk of spontaneous mutations in p53.
Tubal ligation and hysterectomy
Interruption of the female genital tract, by either bilateral tubal ligation or hysterectomy, has been shown to diminish the risk of ovarian cancer.86, 87 In a prospective study of 121,700 registered nurses in the Nurses’ Health Study with 12-year follow-up, a strong inverse relationship between bilateral tubal ligation and ovarian cancer was reported (RR 0.33; 95% CI 0.16–0.64).86 Similarly, in a case–control study of 300 cases and 606 controls, Cornelison and coworkers88 showed a 50% risk reduction in women who had tubal ligations. Hysterectomies seem to reduce the risk of ovarian cancer by 50%.86, 87, 88, 89 It has been hypothesized that tubal ligations and hysterectomies decrease the risk of ovarian cancer by preventing the ascending migration of potential carcinogens. Interestingly, data have shown that tubal ligation reduces the risk of ovarian cancer in BRCA1 but not BRCA2 carriers, and that this effect is magnified in carriers who used oral contraceptives.90
ACKNOWLEDGMENT
The author expresses his appreciation to Dr Cheung Wong, for his contribution to previous editions of this chapter.
REFERENCES
National Cancer Institute: Surveillance, Epidemiology, End Results. National Cancer Institute, 1973–1990 |
|
Coleman MP, Esteve J, Damiecki P et al: Trends in Cancer Incidence and Mortality. Lyon, International Agency for Research on Cancer, 1993 |
|
Jemal A, Thomas A, Murray T et al: Cancer statistics, 2002. CA Cancer J Clin 53: 23, 2002 |
|
Longo DL, Young RC: Cosmetic talc and ovarian cancer. Lancet 2: 349, 1979 |
|
Heller DS, Westhoff C, Gordon RE et al: The relationship between perineal cosmetic talc usage and ovarian talc particle burden. Am J Obstet Gynecol 174: 1507, 1996 |
|
Heller DS, Gordon RE, Westhoff C: Asbestos exposure and ovarian fiber burden. Am J Ind Med 29: 435, 1996 |
|
Henderson WJ, Joslin CA, Turnbull AC et al: Talc and carcinoma of the ovary and cervix. J Obstet Gynaecol Br Commonw 78: 266, 1971 |
|
Whittemore AS, Wu ML, Paffenbarger RS Jr et al: Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol, and coffee. Am J Epidemiol 128: 1228, 1988 |
|
Cramer DW, Welch WR, Scully RE et al : Ovarian cancer and talc: a case-control study. Cancer 50: 372, 1982 |
|
Wong C, Hempling RE, Piver MS, et al: Perineal talc exposure and subsequent epithelial ovarian cancer: a case-control study. Obstet Gynecol 93: 372, 1999 |
|
Harlow BL, Cramer DW, Bell DA et al: Perineal exposure to talc and ovarian cancer risk. Obstet Gynecol 80: 19, 1992 |
|
Primary care and cancer. 21: 20, 2001 |
|
Cramer DW, Welch WR, Hutchison GB et al: Dietary animal fat in relation to ovarian cancer risk. Obstet Gynecol 63: 833, 1984 |
|
Shu XO, Gao YT, Yuan JM et al: Dietary factors and epithelial ovarian cancer. Br J Cancer 59:92, 1989 |
|
Helzlsouer KJ, Alberg AJ, Norkus EP et al: Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst 88: 32, 1996 |
|
Xu YK, Ng WG, Kaufman FR et al: Galactose metabolism in human ovarian tissue. Pediatr Res 25: 151, 1989 |
|
Mangeot M, Djabal A, Myara I et al: Metabolic studies of a sustained galactose overload in rat. Ann Nutr Metab 31: 333, 1987 |
|
Chen YT, Mattison DR, Feigenbaum L et al: Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science 214: 1145, 1981 |
|
Swartz WJ, Mattison DR: Galactose inhibition of ovulation in mice. Fertil Steril 49: 522, 1988 |
|
Kaufman FR, Donnell GN, Roe TF et al: Gonadal function in patients with galactosaemia. J Inherit Metab Dis 9: 140, 1986 |
|
Cramer DW, Harlow BL, Willett WC et al: Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet 2: 66, 1989 |
|
Risch HA, Jain M, Marrett LD et al: Dietary lactose intake, lactose intolerance, and the risk of epithelial ovarian cancer in southern Ontario (Canada). Cancer Causes Control 5: 540, 1994 |
|
Fairfield KM, Hunter DJ, Colditz GA et al: A prospective study of dietary lactose and ovarian cancer. J Gen Intern Med 15: 205, 2000 |
|
Mettlin CJ, Piver MS: A case-control study of milk-drinking and ovarian cancer risk. Am J Epidemiol 132: 871, 1990 |
|
Prentice RL, THomson CA, Hubbell FA et al: Low-fat dietary pattern and cancer incidence in the women's health initiative dietary modification randomized controlled trial. J Natl Cancer Inst 99: 1534, 2007 |
|
Rossing MA, Darling JR, Weiss NS et al: Ovarian tumors in a cohort of infertile women. N Engl J Med. 331: 771, 1994 |
|
Whittemore AS, Wu ML, Paffenberger RS et al: Epithelial ovarian cancer and the ability to conceive. Cancer Res 49: 4047, 1989 |
|
Rossing MA: Ovarian cancer: A risk of infertility treatment. Hum Reprod 11: 46, 1996 |
|
Shushan A, Paltiel O, Iscovich J et al: Human menopausal gonadotropin and the risk of epithelial ovarian cancer. Fertil Steril 65: 13, 1996 |
|
Ness RB, Cramer DW, Goodman MT et al: Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol 155: 217, 2002 |
|
Ross RK, Paganini-Hill A, Mack TM et al: Menopausal oestrogen therapy and protection from death from ischaemic heart disease. Lancet 1: 858, 1981 |
|
Ettinger B, Genant HK, Cann CE: Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 102: 319, 1985 |
|
Farish E, Fletcher CD, Hart DM et al: The effects of conjugated equine oestrogens with and without a cyclical progestogen on lipoproteins, and HDL subfractions in postmenopausal women. Acta Endocrinol (Copenh) 113: 123, 1986 |
|
Whittemore AS, Harris R, Itnyre J, and the Collaborative Ovarian Cancer Group: Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol 136: 1184, 1992 |
|
Hildreth NG, Kelsey JL, LiVolsi VA et al: An epidemiologic study of epithelial carcinoma of the ovary.Am J Epidemiol 114: 398, 1981 |
|
Kaufman DW, Kelly JP, Welch WR et al: Noncontraceptive estrogen use and epithelial ovarian cancer. Am J Epidemiol 130: 1142, 1989 |
|
Cramer DW, Hutchison GB, Welch WR et al: Determinants of ovarian cancer risk. I. Reproductive experiences and family history. J Natl Cancer Inst 71: 711, 1983 |
|
Risch HA: Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol 63: 254, 1996 |
|
Hempling RE, Wong C, Piver MS et al: Hormone replacement therapy as a risk factor for epithelial ovarian cancer: results of a case-control study. Obstet Gynecol 89: 1012, 1997 |
|
Rodriguez C, Calle EE, Coates RJ et al: Estrogen replacement therapy and fatal ovarian cancer. Am J Epidemiol 141: 828, 1995 |
|
Lacey JV, Mink PJ, Lubin JH et al: Menopausal hormone Replacement Therapy and Risk of Ovarian cancer. JAMA 288: 334, 2002 |
|
Million Women Study Collaborators: Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 369: 1703, 2007 |
|
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321-33. |
|
Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002 Jul 17;288(3):334-41 |
|
Zhou B, Sun Q, Cong R et al: Hormone replacement therapy and ovarian cancer risk: A meta-analysis. Gynecol Oncol 108: 641, 2008 |
|
Weinberg RA: The genetic origins of human cancer. Cancer 61: 1963, 1988 |
|
Sager R: Tumor suppressor genes: the puzzle and the promise. Science 246: 1406, 1989 |
|
Knudson AG, Strong LC: Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet 24: 514, 1972 |
|
Gallion HH, Guarino A, DePriest PD et al: Evidence for a unifocal origin in familial ovarian cancer. Am J Obstet Gynecol 174: 1102, 1996 |
|
Trent JM, Kaneka Y, Mitelman F: Report of the committee on structural chromosome changes in neoplasia. Cytogenet Cell Genet 51: 533, 1989 |
|
Hunter T: Cooperation between oncogenes. Cell 64: 249, 1991 |
|
Bishop JM: Molecular themes in carcinogens. Cell 64: 235, 1991 |
|
Marshall CJ: Tumor suppressor genes. Cell 64: 313, 1991 |
|
Filmus JE, Buick RN: Stability of c-K-ras amplification during progression in a patient with adenocarcinoma of the ovary. Cancer Res 45: 4468, 1985 |
|
Schreiber G, Dubeau L: C-myc proto-oncogene amplification detected by polymerase chain reaction in archival human ovarian carcinomas. Am J Pathol 137: 653, 1990 |
|
Berchuck A, Kamel A, Whitaker R, et al: Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res 50: 4087, 1990 |
|
Lurain JR, Piver MS: Familial ovarian cancer. Gynecol Oncol 8: 185, 1979 |
|
Piver MS, Barlow JJ, Sawyer DF: Familial ovarian cancer: increasing in frequency? Obstet Gynecol 60: 397, 1982 |
|
Piver MS, Mettlin CJ, Tsukada Y et al: Familial Ovarian Cancer Registry. Obstet Gynecol 64: 195, 1984 |
|
Lynch HT, Bewtra C, Lynch JF: Familial ovarian carcinoma. Clinical nuances. Am J Med 81: 1073, 1986 |
|
Troche V, Hernandez E: Neoplasia arising in dysgenetic gonads. Obstet Gynecol Surv 41: 74, 1986 |
|
Dozois RR, Kempers RD, Dahlin DC et al: Ovarian tumors associated with the Peutz-Jeghers syndrome. Ann Surg 172: 233, 1970 |
|
Berlin NI, Van Scott EJ, Clendenning et al: Basal cell nevus syndrome. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med 64: 403, 1966 |
|
Hall JM, Lee MK, Newman B et al: Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250: 1684, 1990 |
|
Miki Y, Swensen J, Shattuck-Eidens D et al: A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266: 66, 1994 |
|
Narod S, Smith S, Ponder B et al: An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet 56: 254, 1995 |
|
Steichen-Gersdorf E, Gallion HH, Ford D et al: Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet 55: 870, 1994 |
|
Richards WE, Gallion HH, Schmitschmidt JP et al: BRCA1-related and sporadic ovarian cancer in the same family: implications for genetic testing. Gynecol Oncol 75: 468, 1999 |
|
Wooster R, Neuhausen S, Mangion J et al: Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 265: 2088, 1994 |
|
Ford D, Easton DF, Stratton M et al: Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62: 676, 1998 |
|
Aarnio M, Sankila R, Pukkala E et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81: 214, 1999 |
|
Malander S, Rambech E, Kristoffersson U et al: The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol 101: 238, 2006 |
|
Tobacman JK, Tucker MA, Kase R et al: Intra-abdominal carcinomatosis after prophylactic oophorectomy in ovarian-cancer-prone families. Lancet 2: 795, 1982 |
|
Chen KT, Schooley JL, Flam MS: Peritoneal carcinomatosis after prophylactic oophorectomy in familial ovarian cancer syndrome. Obstet Gynecol 66(suppl): 93S, 1985 |
|
Piver MS, Jishi MF, Tsukada Y et al: Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer 71: 2751, 1993 |
|
Gross TP, Schlesselman JJ: The estimated effect of oral contraceptive use on the cumulative risk of epithelial ovarian cancer. Obstet Gynecol 83: 419, 1994 |
|
Narod SA, Risch H, Moslehi R et al: Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med 339: 424, 1998 |
|
Whittemore AS, Balise RR, Pharoah PDP et al: Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer 91: 1911, 2004 |
|
Purdie D, Green A, Bain C et al: Reproductive and other factors and risk of epithelial ovarian cancer: An Australian case-control study. Int J Cancer 62: 678, 1995 |
|
Risch HA, Marrett LD, Howe GR: Parity, contraception, infertility and the risk of epithelial ovarian cancer. Am J Epidemiol 140: 585, 1994 |
|
Hankinson SE, Colditz GA, Hunter DJ et al: A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer 76: 284, 1995 |
|
Garder WU: Hormonal imbalances in tumorigenesis. Cancer Res 8: 397, 1948 |
|
Cramer DW, Welch WR: Determinants of ovarian cancer risk: Part II. Inferences regarding pathogenesis. J Natl Cancer Inst 71: 717, 1983 |
|
Fathala MR: Incessant ovulation: A factor in ovarian neoplasia. Lancet 2: 163, 1971 |
|
Berchuck A, Bastos E, Schildkraut J: Association between overexpression of p53 and lifetime ovulatory cycles in ovarian cancer (abstract). Gynecol Oncol 64: 288, 1997 |
|
Hankinson SE, Hunter DJ, Colditz GA et al: Tubal ligation, hysterectomy and risk of ovarian cancer: A prospective study. JAMA 270: 2813, 1993 |
|
Parazzini F, Negri E, La Vecchia C et al: Hysterectomy, oophorectomy and subsequent ovarian cancer risk. Obstet Gynecol 81: 363, 1993 |
|
Cornelison TL, Natarajan N, Piver MS et al: Tubal ligation and risk of ovarian cancer. Cancer Detect Prev 21: 86, 1997 |
|
Irwin KL, Weiss NS, Lee NC et al: Tubal sterilization, hysterectomy and subsequent occurrence of epithelial ovarian cancer. Am J Epidemiol 134: 362, 1991 |
|
Narod SA: Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer 2: 113, 2002 |